The quality testing of medical device packaging is highly significant in ensuring the safe delivery of medical devices from its place of origin to the market. Guidance documents such as ISO 11607 provide a list of methods that have the capability to improve the package quality testing
of today’s capability. PTI offers inspection methods that provide more reliable, sensitive and traceable data. We offer in-depth feasibility studies, test method development and test equipment to bring the supreme level of quality to the high-value medical device applications. PTI’s inspection solutions are unique in their performance, reliability and use of sensory technology.
The type of packages for class III medical devices vary greatly in their materials and design. Various factors like size, shape, profile, irregularities, density, weight and configuration are taken into consideration while deciding the package type for medical devices.
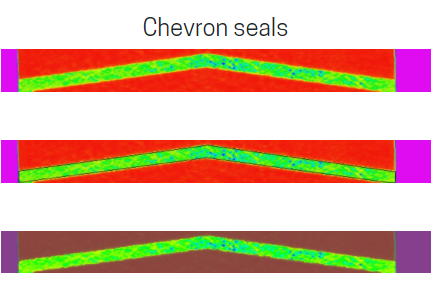
Analysis of FDA letters shows that a vast majority of issues are related to the seal defects of the medical device packaging. PTI’s airborne ultrasound is a non-destructive physical inspection method to assure the quality of package seals both offline for in-depth seal quality analysis and for 100% online pouch seal inspection. This ultrasound technology is a true non-destructive method effective for Tyvek® packaging and is also an ASTM Test Method ASTM F-3004-13. This Test Method is also an FDA Recognized Consensus Standard for Evaluation of Seal Quality and Integrity Using Airborne Ultrasound. The ASTM F-3004-13 Test Method was approved based on the PTI sponsored inter-laboratory research program and is one of the most effective methods for medical device package seal inspection. Airborne ultrasound technology is also referenced in the USP <1207> Chapter guidance for deterministic test methods.
PTI’s vacuum-based leak detection systems offer the ability to inspect both the entire body of the package and seals for micro leaks and defects. VeriPac inspection systems utilize an ASTM approved vacuum decay leak test method (F2338) recognized by the FDA as a consensus standard for package integrity testing. This test method was developed using PTI’s VeriPac leak test instruments. Vacuum decay technology is also referenced in the USP <1207> Chapter Guidance as a deterministic test method for package integrity testing.
The PTI team of scientists and engineers have extensive industry expertise in offering an entire solution that includes test method development and equipment validation.